Aluminum Welding Guide
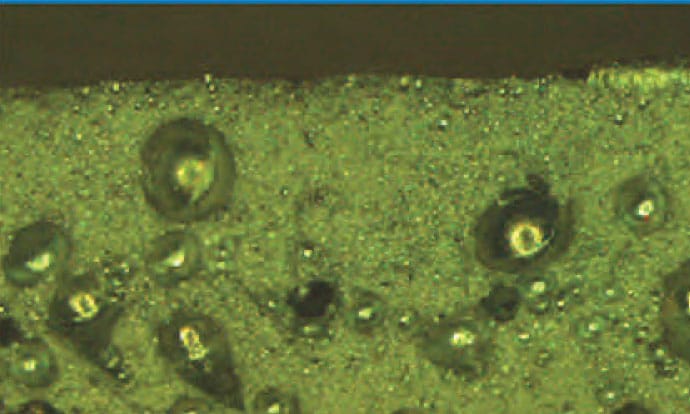
Problem Solving – Weld Joint Porosity
Porosity — Cavity-type discontinuity formed by gas entrapment during solidification.
Producing a weld with low porosity is the responsibility of both the electrode manufacturer and the welder. The electrode manufacturer must supply an electrode that is contamination free and has been tested to show that it is indeed capable of meeting the low porosity standards of AWS A5.10. The welder must incorporate the practices and procedures of codes like AWS D1.2 to ensure that porosity is not introduced into the weld pool. Before welding, process engineers must determine which porosity standard of the applicable code the welded structure is required to meet.
All weld porosity results from the absorption of hydrogen during melting and the expulsion of hydrogen during solidification of the weld pool. The solubility of hydrogen in aluminum increases dramatically after the material reaches its liquid stage. When the aluminum is taken to temperatures above its melting point it becomes very susceptible to hydrogen absorption (see hydrogen solubility chart below). The hydrogen can then form bubbles in the molten aluminum as it solidifies and these bubbles are then trapped in the metal causing porosity.
The cause of porosity in aluminum is hydrogen. The sources of hydrogen that create porosity are:
- Hydrocarbons – In the form of paint, oil, grease, other lubricants and contaminants
- Hydrated aluminum oxide – Aluminum oxide can absorb moisture and become hydrated – the hydrated oxide will release hydrogen when subjected to heat during welding
- Moisture (H2O) – Moisture within the atmosphere can be a serious cause of porosity under certain circumstances – see the calculation of dew point table on page 25. Moisture from other external sources such as compressed air, contaminated shielding gas or from pre-cleaning operations must also be considered.
Note: Hydrogen gas from these sources can become trapped within the weld deposit and create porosity